Track and Trace – providing confidence for the patient and control of costs and counterfeiting for the supplier.
from point of production to point of care, traceability is
being recognised as crucially important, ensuring that
every patient gets the right quantity of the right drug
from the known supplier.
Not only does traceability contribute to product safety and security, but it is also an essential ingredient in improving productvity and profitability while helping to reduce counterfeiting.
(One important contribution of track and trace will be in dealing with the problem of counterfeit drugs. Back in 2010, counterfeit drug sales already exceeded $75 billion worldwide and that number is thought to be well over $100 billion today)
Read more
Governments worldwide are introducing Legislation around Track & Trace….

Across the world, governments are working on legislation to enable seamless tracking and tracing of each single pack of medication from the manufacturer to the point of dispensing the drug to a patient – achieved through serialisation of each and every individual packaging unit.
Ultimately, a consumer or pharmacist will have the ability to take a single package of any drug, scan it and get the information in order to determine whether the package is genuine.
In the USA, pharmacies must be able to capture and maintain transaction information (TI), transaction history (TH), and a transaction statement (TS), sometimes referred to as “the three Ts”, for six years from the date of the transaction for each product received.
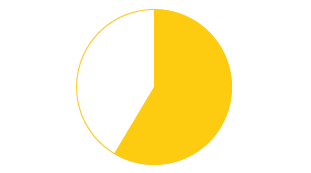
66% of pharma manufacturers surveyed are not serialisation ready
It all starts with Global Serialisation
Global Serialisation and Compliance provides the key to end-to-end transparency, supply chain logistics management and product authentication services across even the most complex supply chains. Serialisation is being understood as the foundation for meeting emerging global regulatory requirements.
The US federal regulation is leading the world with its initial deadline for serialisation in 2017, by which time, any US pharmacy will be able to authenticate the e-Pedigrees of all packages that they receive.
Across the rest of the USA the DSCSA is rolling out. In Europe, serialisation, seen as the first step in the drive to complete traceability, is to be in place for 2019 – European Union’s Directive 2011/62/EU
Other countries have also announced their serialisation requirements. India, China (SFDA DMF), Argentina, Brazil (Anvisa RDC-54), Turkey and South Korea..
How compliant are you?
2D Data Matrix coding proves both effective and inexpensive

The Industry preference is to print or code at the packaging line. The technique allows more control which is particularly important in high-speed operations.
When it comes to cost, printed 2D Data Matrix codes are proving the most cost effective.
Serialisation, the starting point of the track-and-trace process, means to make each product unique by placing an item identifier (serial number) on every single product which can then be traced right through the supply chain. The 2D Data Matrix is applied to every item on the production line enabling all subsequent product-related events, such as “produced,” “shipped,” “received,” “dispensed” to be captured along the supply chain by scanning the Data Matrix identifier. This captured information is then stored in a database to be integrated with product-related information – created as and where the product was prepared.
Videojet provides serialisation to fit in with your production
Videojet offers a unique range of novel coding solutions for the wide range of packaging form factors in pharmaceuticals.
Coding on vials is an excellent example, bearing in mind their small size and the complex sequence of packaging operations. Videojet inkjet marking provides a clear and rugged “on-vial” traceability code to ensure the integrity of the data throughout the packaging process.
Videojet fiber laser marking systems also offer an ideal solution for high-speed marking on robust plastic and metal packaging materials.

PRODUCT RECOMMENDATION
Videojet Laser Product Range Brochure
High Density Polyethylene (HDPE) bottles remain one of the leading packaging solutions for pharmaceutical products. While many HDPE bottles are labelled, Videojet offers technology that makes it possible to mark direct to the bottle itself, rather than the label, in order to provide reliable and permanent traceability.
Matrix code on the bottom of a bottle can simplify downstream machine vision reading by eliminating the need to orient the bottle.
Using the Videojet UV laser at the targeted wavelength creates a dark, permanent, high resolution Data Matrix code against the light coloured HDPE substrate.

PRODUCT RECOMMENDATION
The Videojet 7810 UV laser
Videojet offers a range of products and support to deliver high quality traceability codes for the full range of pharmaceutical packaging. Advantaged printers coupled with a broad portfolio of inks produce high quality Data Matrix codes.
Long-standing relationships with pharmaceutical OEMs allow Videojet to ensure its printers are properly integrated as part of a full system.
The antidote for your high R&D costs:
low cost of ownership
The Videojet 1000 Line of small character inkjet printers provide 99.8% availability,
enabling greater customer productivity and reduced maintenance costs.

